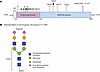
Epithelial outgrowth through mesenchymal rings drives alveologenesis
With real-time, long-term 4-dimensional imaging of living lung slices, Negretti et al. developed a model of lung formation of epithelial ballooning through contractile mesenchymal rings. The cover image is a still frame from an 80-hour 4-dimensional live-imaging experiment of a precision cut lung slice from a postnatal day 5 mouse, at the saccular-alveolar transition. Alveolar type 2 cells are shown in cyan, and other cells are shown in magenta. Image credit: Nicholas Negretti and Jennifer Sucre.
In-Press Preview - More
Abstract
Type 1 diabetes (T1D) is precipitated by the autoimmune destruction of the insulin-producing β-cells in the pancreatic islets of Langerhans. Chemokines have been identified as major conductors of islet infiltration by autoaggressive leukocytes, including antigen-presenting cells and islet autoantigen-specific T cells. We have previously generated a roadmap of gene expression in the islet microenvironment during T1D in a mouse model and found that most of the chemokine axes are chronically upregulated during T1D. The XCL1/XCR1 chemokine axis is of particular interest, since XCR1 is exclusively expressed on conventional dendritic cells type 1 (cDC1) that excel by their high capacity for T cell activation. Here we demonstrate cDC1 expressing XCR1 are present in and around the islets of patients with T1D and of islet-autoantibody positive individuals. Further, we show that XCL1 plays an important role in the attraction of highly potent dendritic cells expressing XCR1 to the islets in an inducible mouse model for T1D. XCL1-deficient mice display a diminished infiltration of XCR1+ cDC1 and subsequently a reduced magnitude and activity of islet autoantigen-specific T cells resulting in a profound decrease in T1D incidence. Interference with the XCL1/XCR1 chemokine axis might constitute a novel therapy for T1D.
Authors
Camilla Tondello, Christine Bender, Gregory J. Golden, Deborah Puppe, Elisa Blickberndt, Monika Bayer, Giulia K. Buchmann, Josef Pfeilschifter, Malte Bachmann, Edith Hintermann, Ralf P. Brandes, Michael R. Betts, Richard A. Kroczek, Urs Christen
Abstract
While immune checkpoint inhibition (CPI) has reshaped cancer treatment, the majority of cancer patients do not benefit from this approach, which can also cause immune-related adverse events. Induction of IFNγ responses is thought be necessary for anti-tumor immunity, but growing evidence also implicates IFNγ as a tumor-intrinsic mediator of CPI resistance. CPI-induced IFNγ mediates activation-induced cell death in T cells as an immune-intrinsic mechanism of resistance. In this study, we show that transient block of IFNγ signaling through administration of the JAK1 inhibitor ABT-317 enhances anti-tumor T cell responses with CPI in pre-clinical models. Importantly, sequential but not concomitant ABT-317 treatment led to significantly reduced toxicity and improved tumor efficacy. Sequential treatment reduced activation-induced T cell death and enhanced expansion of tumor-reactive T cell subsets with increased effector function in vivo and ex vivo. Only CPI in combination with ABT-317 also enhanced memory responses by protecting mice from tumor rechallenge. These results demonstrate that JAK inhibition within a discrete time window following CPI addresses an immune-intrinsic mechanism of therapeutic resistance.
Authors
Marcel Arias-Badia, PeiXi Chen, Yee May Lwin, Aahir Srinath, Aram Lyu, Zenghua Fan, Serena S. Kwek, Diamond N. Luong, Ali Setayesh, Mason Sakamoto, Matthew Clark, Averey Lea, Rachel M. Wolters, Andrew Goodearl, Fiona A. Harding, Jacob V. Gorman, Wendy Ritacco, Lawrence Fong
Abstract
Chlamydia trachomatis (CT) is the most common bacterial sexually transmitted infection globally. Understanding natural immunity to CT will inform vaccine design. This study aimed to profile immune cells and associated functional features in CT-infected women, and determine immune profiles associated with reduced risk of ascended endometrial CT infection and CT reinfection. PBMCs from CT-exposed women were profiled by mass cytometry and random forest models identified key features that distinguish outcomes. CT+ participants exhibited higher frequencies of CD4+ Th2, Th17, and Th17 DN CD4 T effector memory (TEM) cells than uninfected participants with decreased expression of T cell activation and differentiation markers. Minimal differences were detected between women with or without endometrial CT infection. Participants who remained follow-up negative (FU-) showed higher frequencies of CD4 T central memory (TCM) Th1, Th17, Th1/17, and Th17 DN but reduced CD4 TEM Th2 cells than FU+ participants. Expression of markers associated with central memory and Th17 lineage were increased on T cell subsets among FU- participants. These data indicate that peripheral T cells exhibit distinct features associated with resistance to CT reinfection. The highly plastic Th17 lineage appears to contribute to protection. Addressing these immune nuances could promote efficacy of CT vaccines.
Authors
Kacy S. Yount, Chi-Jane Chen, Avinash Kollipara, Chuwen Liu, Neha Vivek Mokashi, Xiaojing Zheng, C Bruce Bagwell, Taylor B. Poston, Harold C. Wiesenfeld, Sharon L. Hillier, Catherine M. O'Connell, Natalie Stanley, Toni Darville
Abstract
The role of mesenchymal cells during respiratory infection is not well defined, including whether, which, and how the different types of mesenchymal cells respond. We collected all mesenchymal cells from lung single-cell suspensions of mice that were naïve (after receiving only saline vehicle), pneumonic (after intratracheal instillation of pneumococcus 24 hours previously), or resolved from infection (after non-lethal pneumococcal infections 6 weeks previously) and performed single-cell RNA sequencing. Cells clustered into five well-separated groups based on their transcriptomes: matrix fibroblasts, myofibroblasts, pericytes, smooth muscle cells, and mesothelial cells. Fibroblasts were the most abundant and could be further segregated into Pdgfra+Npnt+Ces1d+Col13a1+ alveolar fibroblasts and Cd9+Pi16+Sca1+Col14a1+ adventitial fibroblasts. The cells from naïve and resolved groups overlapped in dimension reduction plots, suggesting the mesenchymal cells returned to baseline transcriptomes after resolution. During pneumonia, all mesenchymal cells responded with altered transcriptomes, revealing a core response that had been conserved across cell types as well as distinct mesenchymal cell type-specific responses. The different subsets of fibroblasts induced similar gene sets, but the alveolar fibroblasts responded more strongly than the adventitial fibroblasts. These data demonstrated diverse and specialized immune activities of lung mesenchymal cells during pneumonia.
Authors
Alicia M. Soucy, Jourdan E. Brune, Archana Jayaraman, Anukul T. Shenoy, Filiz T. Korkmaz, Neelou S. Etesami, Bradley E. Hiller, Ian M.C. Martin, Wesley N. Goltry, Catherine T. Ha, Nicholas A. Crossland, Joshua D. Campbell, Thomas G. Beach, Katrina E. Traber, Matthew R. Jones, Lee J. Quinton, Markus Bosmann, Charles W. Frevert, Joseph P. Mizgerd
Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD)—characterized by excess accumulation of fat in the liver—now affects one third of the world’s population. As MASLD progresses, extracellular matrix components including collagen accumulate in the liver causing tissue fibrosis, a major determinant of disease severity and mortality. To identify transcriptional regulators of fibrosis, we computationally inferred the activity of transcription factors (TFs) relevant to fibrosis by profiling the matched transcriptomes and epigenomes of 108 human liver biopsies from a deeply characterized cohort of patients spanning the full histopathologic spectrum of MASLD. CRISPR-based genetic knockout of the top 100 TFs identified ZNF469 as a regulator of collagen expression in primary human hepatic stellate cells (HSCs). Gain- and loss-of-function studies established that ZNF469 regulates collagen genes and genes involved in matrix homeostasis through direct binding to gene bodies and regulatory elements. By integrating multiomic large-scale profiling of human biopsies with extensive experimental validation we demonstrate that ZNF469 is a transcriptional regulator of collagen in HSCs. Overall, these data nominate ZNF469 as a previously unrecognized determinant of MASLD-associated liver fibrosis.
Authors
Sebastian Steinhauser, David Estoppey, Dennis P. Buehler, Yanhua Xiong, Nicolas Pizzato, Amandine Rietsch, Fabian Wu, Nelly Leroy, Tiffany Wunderlin, Isabelle Claerr, Philipp Tropberger, Miriam Müller, Alexandra Vissieres, Lindsay M. Davison, Eric H. Farber-Eger, Quinn S. Wells, Quanhu Sheng, Sebastian Bergling, Sophia A Wild, Pierre Moulin, Jiancong Liang, Wayne J. English, Brandon Williams, Judith Knehr, Marc Altorfer, Alejandro Reyes, Johannes Voshol, Craig Mickanin, Dominic Hoepfner, Florian Nigsch, Mathias Frederiksen, Charles R. Flynn, Barna D. Fodor, Jonathan D. Brown, Christian Kolter